2255-tetramethylhexane 150 This plot of molar mass vs. For example part b in Figure 2124 shows 22-dimethylpropane neopentane and n-pentane both of which have the empirical formula C 5 H 12.

Structural Formula Of 2 2 Dimethyl Propane Brainly In
100 23 ratings there only one p.
. 22-dimethyl-1-propanol - cas 75-84-3 synthesis structure density melting point boiling point. Draw the structure of 2 2 dimethylpropane. 5 n_C val 12 n_H val 32 Calculate the number of electrons needed to completely fill the valence shells for carbon n_C full 8 and hydrogen n_H full 2.
5 n_C full 12 n_H full 64 Subtracting these two numbers shows. Then we number the carbons and attach 2. Draw the structure of each compound named below.
Start by drawing the overall structure of the molecule. A methyl group substitutes the chain at C2. Not affected by aqueous solutions of acids alkalis most oxidizing agents and most reducing agents.
Organic Chemistry IntroductionLearn to draw the structure for 22-dimethylbutane in an easy wayTo write the structure for 22-dimethylbutane we first draw. 100 23 ratings there only one p. 120 0 0 Marvin JS Chem ooooood Submit Request Answer Next Provide Feedback.
This is the best answer based on feedback and ratings. Count the total valence electrons of the carbon n_C val 4 and hydrogen n_H val 1 atoms. Start by drawing the overall structure of the molecule.
In other settings mostly unreactive. Click here to get an answer to your question Draw the structure of 2-2dimethylpropane. The 2 2- dimethylpropane is also known as Neopentane and is one of 3 structural isomers with the structural formula C5H12.
2 2 Dimethyl Propane Structure Brainly In For example part b in Figure 2124 shows 22-dimethylpropane neopentane and n-pentane both of. Draw the structure of hydrocarbons 22 -dimethylpropane - 27125252 anushka0816 anushka0816 anushka0816. Count the total valence electrons of the carbon n_C val 4 and hydrogen n_H val 1 atoms.
5 n_C full 12 n_H full 64 Subtracting these two numbers shows. 22-DIMETHYLPROPANE may be incompatible with strong oxidizing agents like nitric acid. More the steric hindrance slower the reactionThus the order of reactivity will be 1 2 3.
1-Chloro-22-dimethylpropane C5H11Cl CID 12956 - structure chemical names physical and chemical properties classification patents literature biological. Charring may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. Draw the structure s of all of the possible monochloro derivatives of 22-dimethylpropane C5H11Cl.
See the answer See the answer done loading. C 5 H 12 O. PLEASE HELP WITH THIS ORGO CHEM QUESTION thank you.
In other settings mostly unreactive. Rinkudhiman495 rinkudhiman495 25022020 Chemistry Secondary School 5 pts. Not affected by aqueous solutions of acids alkalis most oxidizing agents and most reducing agents.
Draw the structures of all of the possible monochloro derivatives of 22-dimethylpropane C 5 H 11 Cl. 5 n_C val 12 n_H val 32 Calculate the number of electrons needed to completely fill the valence shells for carbon n_C full 8 and hydrogen n_H full 2.

Solved Draw The Structural Formula Of The Following Names Chegg Com

Draw The Lewis Structure And State Which Of The Following Compounds Are Isomers I Pentane Ii 2 Methylbutane Iii 2 3 Dimethylbutane Iv 2 2 Dimethylpropane Study Com

Ch3 C Ch The Iupac Name Of The Compound Is
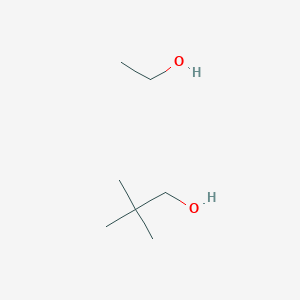
2 2 Dimethylpropan 1 Ol Ethanol C7h18o2 Pubchem
Chemistry Organic Molecules Trends In Boiling Temp Viscosity And Flash Point

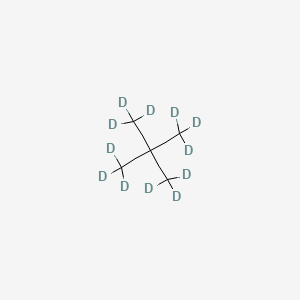
2 2 Dimethylpropane D12 C5h12 Pubchem

How To Draw The Structure For 2 2 Dimethylbutane Alkanes Organic Chemistry Youtube
0 comments
Post a Comment